HOW DOES IT HELP YOU?
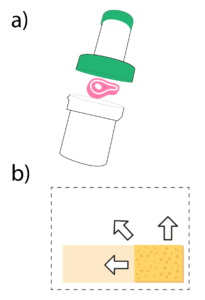
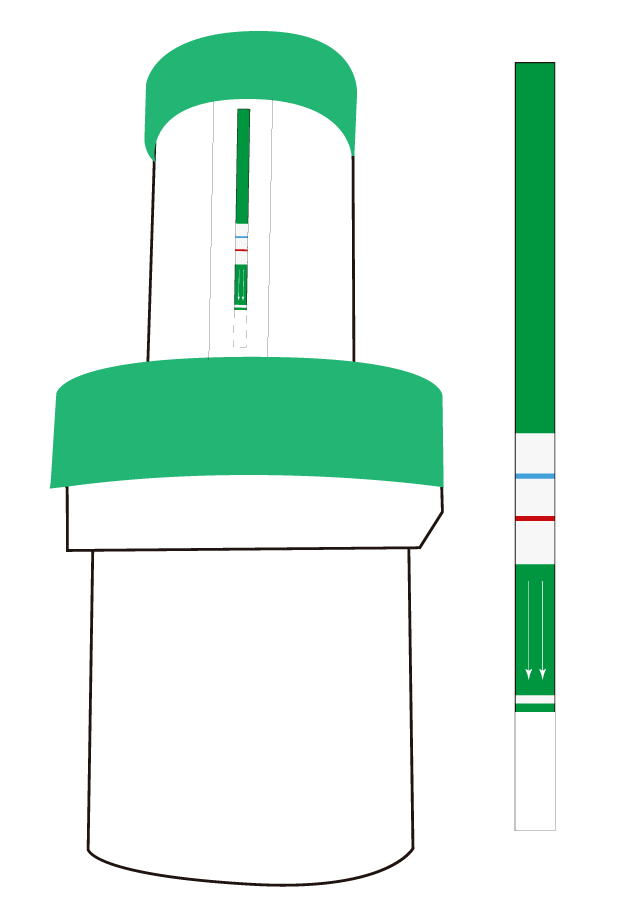
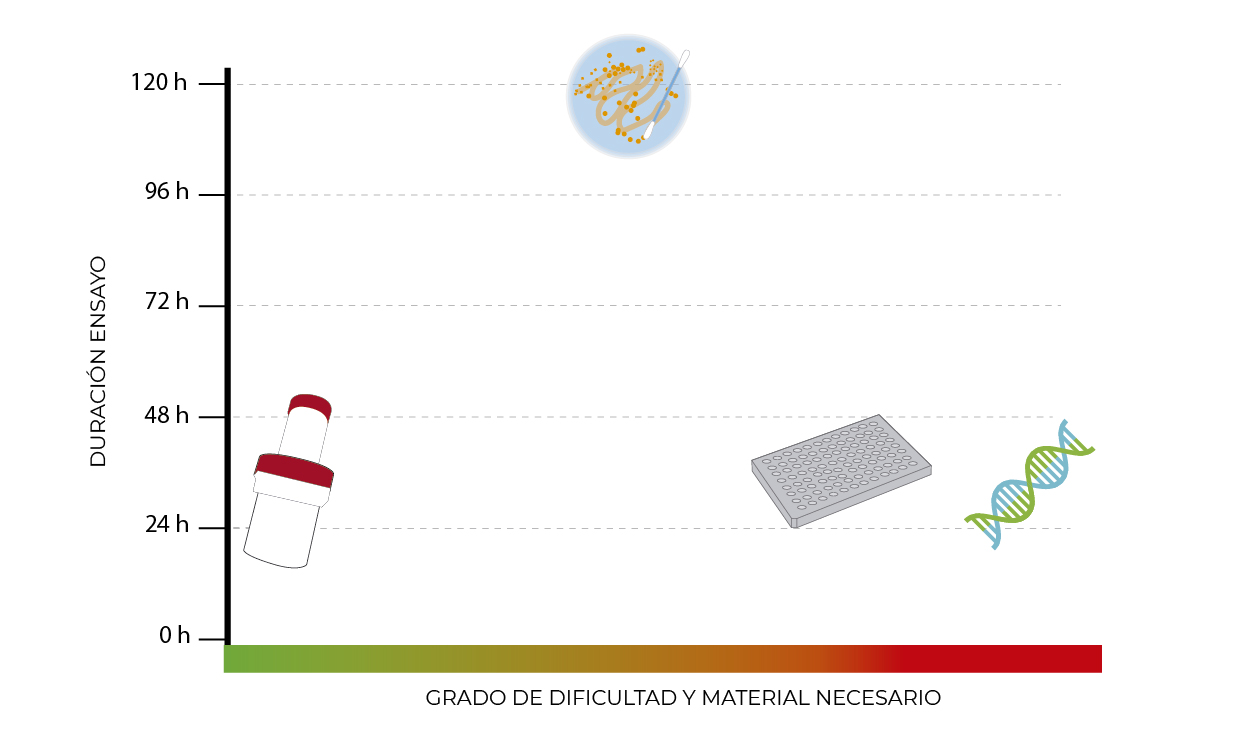
The new device Microlab let you detect the presence of Listeria in food samples and working surfaces in an easy and 100% safe way. MicroLab integrates, in a single disposable device, all steps of the analysis in order to facilitate and speed internally – the results, minimizing losses due to a contamination.