Eclipse FARM-4G & COMET4
One drop of milk, one minute of your time. For an automatic analysis of more than 50 antibiotic substances and with real-time results wherever you need it to.
Do you want to make sure that the milk from your treated cow is free from antibiotics?
Do you need to check that your tank milk , truck tank or silo is free from any antibiotic substance that can interfere with your production?
Can I use transport time to analyse milk before arrival to the plant?
HOW DOES IT HELP YOU?
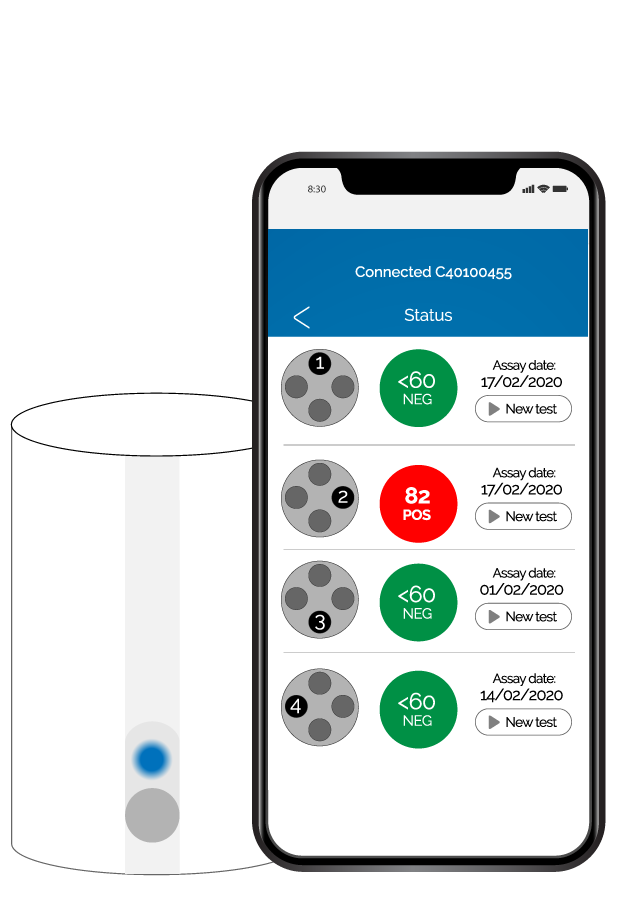
Eclipse Farm 4G & Comet4 is the first system on the market that allows to carry out an automatic antibiotic screening test and share the results in real-time. Just add a drop of milk into the test tube and Comet4 will automatically run the test in less than 2.5 hours. Regardless of where you are, you will receive the results on your smartphone, your cloud account or your email, always in real-time.

GAEC of L’Estiala. Brittany, France. 110 dairy cows
“Nous nous sommes équipé du détecteur d’antibiotiques en Aout 2020. J’utilise le détecteur avec mon lphone por m’assurer de l’absence d’antibiotiques sur les multipares après vêlage mais aussi pour les vaches en fin de traitement. Le coût d’un test revient à moins de 1 euro. La pénalité liée au antibiotiques peut monter jusq’à 500€/1000L à l’échelle de notre élevage. Le détecteur est un outil efficace et facile à utiliser qui nous apporte rigueur et sécurité dans la qualité du lait livré!”
“We equipped ourselves with the antibiotic detector in August 2020. I use the detector with my iPhone to make sure cows are free of antibiotics after calving and at the end of their treatment. The cost of one test is less than 1 euro. The penalty for antibiotics can be up to 500€/1000L for our farm. The detector is an effective and easy-to-use tool that gives us precision and security in the quality of milk we delivered!”

Andrew Smith, Herb Manager, Pearn Wyatt & Son, Norfolk, UK
“I found Comet very useful. I don´t normally test as it takes too much time up, but with the results coming up to my email I don´t have to be at the tester waiting for it to finish. It also picked up positives up that I would have normally put back in the tank.”
APPLICATIONS AND USERS
TANK
TRUCK
PROCESSING PLANT
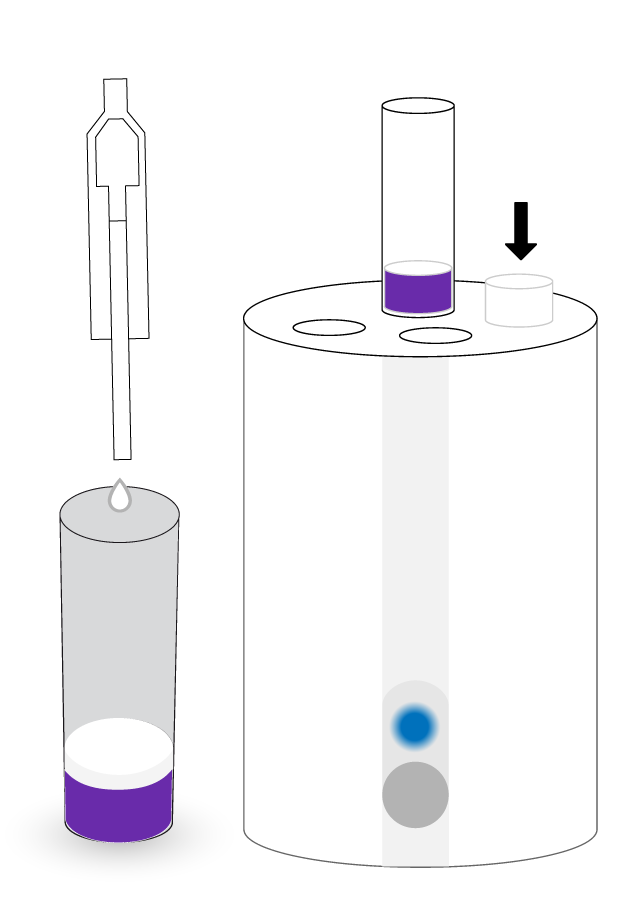
ASSAY PROCEDURE
TEST PRINCIPLE
ECLIPSE test is based on the growth inhibition of the Geobacillus stearothermophilus bacterium. In a specific culture medium and under suitable conditions, the spores of this bacterium germinate and multiply, acidifying the medium, producing a colour change in the culture medium from blue to yellow. If the milk sample contains an antimicrobial concentration higher than the test detection limit, the bacterial growth is inhibited and therefore the colour change of the medium will not occur.
TECHNICAL DATA
- Scope: simultaneous detection of 8 groups of antibiotics in milk (>50 antibiotic substances). Sensitivity adapted to European MRLs
- Assay time: 2 hours 30 min.
- Manual assay: one-stage incubation at 63°C for approximately 2.5h and visual reading.
- Results: numerical results, automatic reading and results are automatically sent (WIFI/Bluetooth/4G)
- Portable: 4 hours battery life
- Format: 25 or 50 tubes
Validations:

Eclipse Farm 4G & Comet were approved by the AOAC-RI Performance Tested Methods (PTM) Program for 29 antibiotics. (PTM License Number 022101)
Validated by ILVO: Eclipse Farm 4G & Comet4 fulfils the Belgian FASFC approval criteria regarding detection capabilities.

Internal validation: according to ISO 13969:2003 and CRL guides (Community Reference Laboratory for veterinary residues), Commission Decision 2002/657/EC.
Legislation:
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin.